The close-packed structure NaxWO3 is a defective ABO3-type perovskite structure. In this structure, O2- and Na+ are arranged in a cubic close-packed arrangement, W and O form a WO6 octahedron and share the vertex O, and Na+ is between the WO6 octahedrons. In the voids of , there is no discrete cation Na+ and oxoacid b anion WO3- in the whole crystal, and it belongs to the compound oxide structure rather than the oxoacid structure. Therefore, it is different from the compound of calcium carbonate structure composed of ABO3. Resistant to all acids except hydrofluoric acid and insoluble in water showing extreme chemical inertness to acids.
The unstable oxidation state of W in NaxWO3 and its reducibility. As mentioned above, the average oxidation number of W in NaxWO3 is between V-VI, X mol W is + V oxidation state, and the most stable oxidation state of W is + VI, which makes NaxWO3 stable under alkaline conditions. With strong reducibility, NaxWO3 is oxidized by air under heating conditions, soluble in strong alkali solution exposed to air, and can also reduce the ammoniated aqueous solution of silver nitrate.
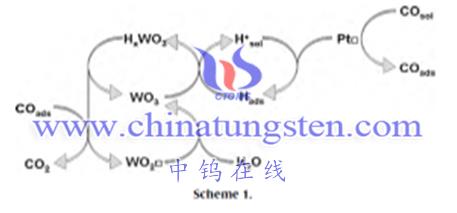
Example: PtWO3/C as a catalyst for the conversion of CO to CO2
More details of cesium tungsten bronze product, please visit website: cesium-tungsten-bronze.com
Please contact CHINATUNGSTEN for inquiry and order of cesium tungsten bronze:
Email: sales@chinatungsten.com
Tel.: 86 592 5129595






