Barium tungsten electrode, also known as tungsten barium alloy electrode, is an electrode made from an alloy material of tungsten and barium. In electrochemical analysis, the application of barium tungsten electrodes is mainly reflected in the following aspects:
Measure the chemical redox potential in water bodies
Barium tungsten electrodes have high potential stability and low capacitance, allowing them to accurately measure the chemical redox potential in water.

Solution electrolysis and electrodeposition experiments
In experiments such as solution electrolysis and electrodeposition, the excellent electrochemical properties and stability of barium tungsten electrodes make them an ideal electrode material.
Electrochemical analysis of samples containing heavy metal ions, halogen elements, and drugs
Barium tungsten electrodes are sensitive to the redox response of many organic and inorganic substances, and can operate over a wide range of pH conditions. Therefore, they are widely used in the electrochemical analysis of samples such as heavy metal ions, halogen elements, and drugs in aqueous solutions.
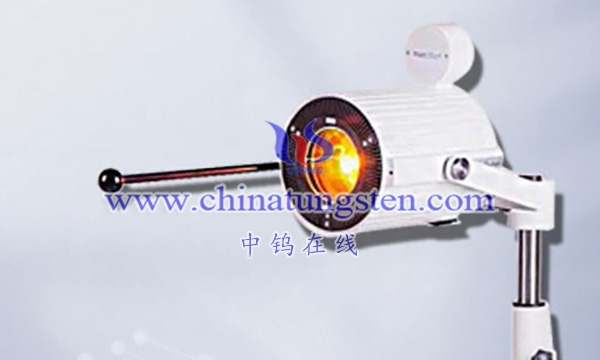
The applications of barium tungsten electrodes are mainly due to their excellent characteristics such as high electron emission ability, low escape work, high electron emission current density, good gas discharge lamp ignition speed, and low electrode operating temperature. In addition, barium tungsten electrodes also have no radioactive toxicity and anti poisoning ability, making them widely applicable in electrochemical analysis.
During operation, it is necessary to pay attention to preventing electrodes from being impacted or mechanically damaged to ensure their performance and accuracy.
More details of tungsten barium elecrode or tungsten barium cathode, please visit website: http://tungsten.com.cn/barium-tungsten-cathode.html
Please contact CHINATUNGSTEN ONLINE for inquiry and order of tungsten needles:
Email: sales@chinatungsten.com
Tel.: +86 592 5129595