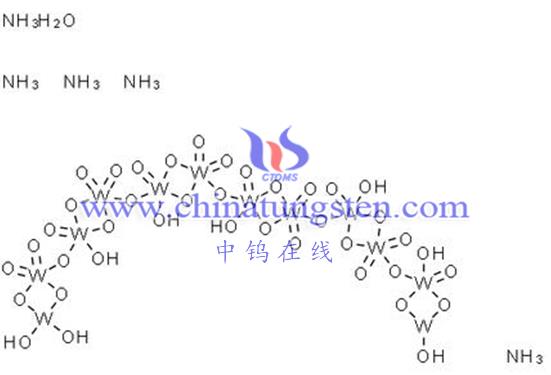
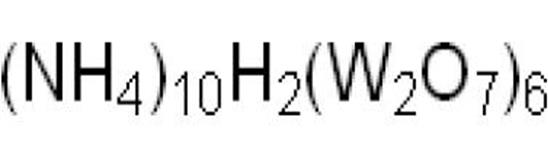The solubility of ammonium metatungstate in water is affected by the following main factors:
Temperature
Temperature is one of the important factors affecting the solubility of ammonium metatungstate.
Generally speaking, as the temperature increases, the solubility of ammonium metatungstate in water increases. For example, at 20°C, the solubility of AMT (ammonium metatungstate) in water is 303.9g/100g H₂O.
The increase in temperature can promote molecular movement, making it easier for solid substances to disperse into the solvent, thereby increasing the solubility.
pH value
The pH value in the solution also affects the solubility of ammonium metatungstate.
In a weakly acidic environment, the solubility of ammonium metatungstate is higher; in a weakly alkaline environment, the solubility is lower.
For example, at a pH of about 7.0, the solubility of ammonium metatungstate is the highest, and when the pH value is greater than 9.0 or less than 5.0, its solubility drops sharply.
Solvent type
Solubility is also affected by the type of solvent.
The solubility of ammonium metatungstate in water is higher than that in organic solvents such as ethanol.
Pressure
Pressure can also affect the solubility of ammonium metatungstate, but since the solubility of ammonium metatungstate is usually very high, the effect of pressure is small and is usually not considered as a major factor.
In summary, the solubility of ammonium metatungstate in water is mainly affected by factors such as temperature, pH value, and solvent type. In practical applications, these factors can be adjusted as needed to control the solubility of ammonium metatungstate to meet different needs.
More details of ammonium metatungstate product, please visit website: http://ammonium-metatungstate.com/
Please contact CHINATUNGSTEN for inquiry and order of ammonium metatungstate:
Email: sales@chinatungsten.com
Tel.: 86 592 5129595