Tungsten trioxide (WO3) can react with hydrogen (H2), and the reduction reaction of hydrogen occurs under high temperature conditions. This reaction can be expressed as: WO3 + 3H2 → W + 3H2O
In this reaction, tungsten trioxide is reduced to tungsten (W) while hydrogen is oxidized to water (H2O). This is a common reduction reaction in which tungsten trioxide is reduced to metallic tungsten by supplying hydrogen. This reaction usually requires high temperature conditions (usually above 500°C) to proceed. In addition, the reaction rate also depends on the reaction conditions and the presence or absence of catalysts. In practical applications, catalysts such as metals such as aluminum or iron are often used to promote the reaction rate and efficiency.
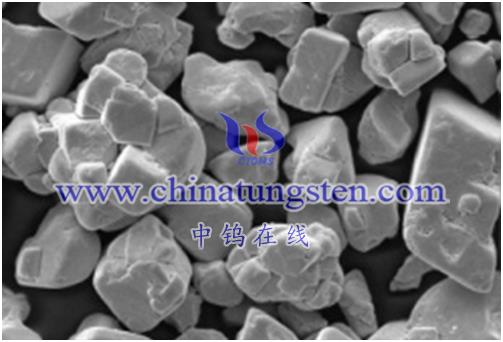
This reaction is of great significance in some applications, such as the preparation of metal tungsten materials, the preparation of catalysts, etc. However, attention needs to be paid to safety and operating conditions, ensuring that it is performed with proper equipment and conditions. When performing this reaction, please refer to the relevant chemical literature and operating instructions to ensure safe and efficient experimental procedures.
More details of tungsten oxide product, please visit website: tungsten-oxide.com
Please contact CHINATUNGSTEN for inquiry and order of tungsten oxide:
Email: sales@chinatungsten.com
Tel.: 86 592 5129595






